Alexandra C. Stenson
Education
- B.S. Florida State University, Chemistry and Environmental Chemistry, 1998
- Ph.D. Florida State University, Analytical Chemistry, 2002
Research
Characterization of unknown compounds from extremely complex mixtures remains one of the major challenges in modern analytical chemistry. Complex mixtures in themselves are not necessarily crippling if enough is known about the components allowing for the design of highly specific separation techniques (this is generally taken advantage of for biological samples). True unknowns are somewhat more difficult, but can generally be structurally identified as long as enough information-rich data are compiled (e.g. NMR, IR, X-ray crystallography and mass spectra). The combination of complexity and true unknowns is, however, still a large stumbling block.
Mixtures too complex to be fractionated into individual components are relatively common in nature. Humic substances (the condensation and degradation products of dead and decaying plant and animal matter) are one such example. Humics are ubiquitous in nature and of great agricultural and environmental importance. More recently biomedical uses for these compounds (particularly as antiviral agents) have also been identified.
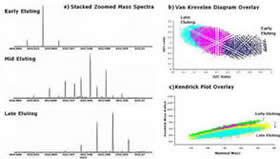
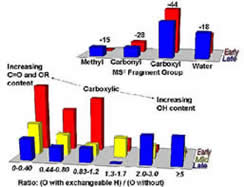
In this research group we are using chromatography and mass spectrometry to characterize humic substances on the molecular level. Research goals involve the development of pre-fractionation methods that reduce the overall complexity of humic mixtures; identification of structural components for individual humic molecules through tandem MS techniques; and mimicking of humic substances through synthetic standards
See MorePublications and Collaborations
-
Brown, T.A.; Jackson, B.A.; Bythell, B.J.; Stenson*, A.C.; Benefits of multidimensional fractionation for the study and characterization of natural organic matter. Journal of Chromatography A (2016), 1470, 84-96. DOI: 10.1016/j.chroma.2016.10.005
-
Connell, M.; Stenson, A.C.; Weinrich, L.; LeChevallier, M.; Boyd, S.B.; Ghosal, R.R.; Dey, R.; Whelton*, A.J.; PEX and PP Water Pipes: Assimilable Carbon, Chemicals, and Odors. Journal - American Water Works Association (2016), 108, E192-E204. DOI: http://dx.doi.org/10.5942/jawwa.2016.108.0016.
-
Harris, B.D.; Brown, T.A.; McGehee, J.L.; Houserova, D.; Jackson, B.A.; Buchel, B.C.; Krajewski, L.C.; Whelton, A.J.; Stenson*, A.C.; Characterization of Disinfection Byproducts from Chromatographically Isolated NOM through High Resolution Mass Spectrometry. Environmental Science & Technology (2015), 49, 14239–14248. DOI: 10.1021/acs.est.5b03466.
-
Kelley, K.M.; Stenson, A.C.; Cooley, R.; Dey, R.; Whelton*, A.J.; The Cleaning Method Selected for New PEX Pipe Installation Can Affect Short-Term Drinking Water Quality. Journal of Water and Health (2015), 13, 960-969. DOI: 10.2166/wh.2015.243.
-
Stenson, A.C.; West, K.N.; Reichert, W.M.; Klomkaew,P,; Cassity, C.G.; Dobyns, B.M.; Siu,B.; Davis*, J.H., Jr.; Multi-cation ionic liquids and a direct, reproducible ‘non-mixing’ way to make them. Chemical Communications (2015), 51, 15914-15916. DOI: 10.1039/c5cc05843k.
-
*Novotny, N.R.; Capley, E.N.; Stenson*, A.C.; Fact or Artifact: The representativeness of ESI-MS for complex natural organic mixtures. Journal of Mass Spectrometry (2014), 49, 316-326.
-
*Stenson*, A.C.; Ruddy, B.M.; Bythell, B.J.; Ion Molecule Reaction H/D exchange as a Probe for Isomeric Fractionation in Chromatographically Separated Natural Organic Matter. International Journal of Mass Spectrometry (2014), 360, 45-53.
-
*Chen, L.; Mullen, G.E.; Le Roch, M.; Cassity, C.G.; Gouault, N.; Fadamiro, H.Y.; Barletta, R.E.; O'Brien, R.A.; Sykora, R.E.; Stenson, A.C.; West, K.N.; Horne, H.E.; Hendrich, J.M.; Xiang, K.R.; Davis*, J.H., Jr.; On the Formation of a Protic Ionic Liquid in Nature. Angewandte Chemie International Edition (2014), 53, 1-5.
Undergraduate Research Information
In this lab, we do analytical chemistry. Analytical chemists are essentially the detectives
of chemistry. We use clever techniques and instrumentation to discover what is in
a sample. The tools we use the most are HPLC (high pressure liquid chromatography)
to separate components of a sample from each other and MS (mass spectrometry) to identify
them. In addition, we use a host of tools and techniques ranging from state-of-the-art
to definitely very old-school (e.g., dialysis, extraction, metal-affinity chromatography,
hydrogen-deuterium exchange, even synthesis, when the situation calls for it). We
frequently have the most fun around here when we are a little bit out of our element
and have to discover how to work something new (or how to fix it).